About Actimed
Pipeline
Disease Overview
News & Events
Contact

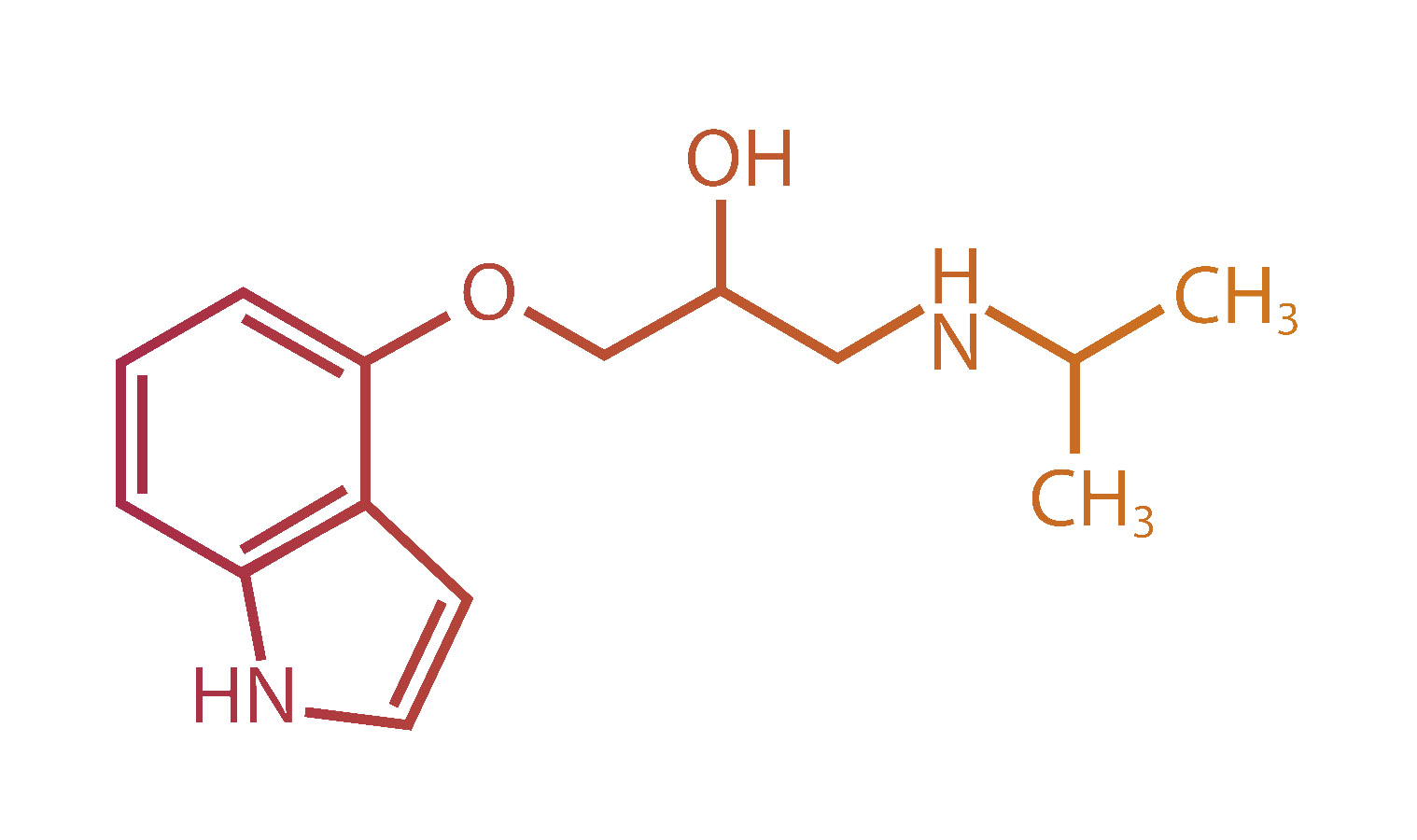
benzoate (ACM-001.1)
Actimed has conducted a Phase 1 Pharmacokinetic and Pharmacodynamic (PK/PD) comparative bioavailability study of S-pindolol benzoate in healthy subjects (NCT06028321). This study was published in the Journal of Cachexia, Sarcopenia and Muscle in December 2024 and demonstrated that S-pindolol benzoate, when administered as the single enantiomer, was essentially bioequivalent to an equivalent dose of S-pindolol when administered as part of a racemic mixture of pindolol (the authorised product). The study also demonstrated the lack of in vivo racemization of S-pindolol to R-pindolol.
Pharmacokinetics, Pharmacodynamics and Bioavailability of ACM-001.1 (S-Pindolol Benzoate) in Healthy Volunteers. Misselwitz, F., Henderson, D., Menakuru, S., Morten, E., Roe, C., Whitaker, G., Wohlfeil, S. and McDermott, J. (2024). The publication may be accessed here.
IMPACT Programme: Treating Cancer Cachexia
Following this successful PK/PD study, a Phase 2b/3 clinical development programme for S-pindolol benzoate in cancer cachexia, the IMPACT Programme (Improving cancer cachexia with ACTAs), is planned to expand upon the positive results demonstrated in an exploratory proof of concept Phase 2a trial for S-pindolol, (ACT-ONE)2.
The IMPACT programme will consist of two trials of identical design: one in patients with non-small cell lung cancer (NSCLC) and the other in colorectal cancer (CRC), both groups with a diagnosis of cachexia. The studies are designated IMPACT-NSCLC and IMPACT-CRC respectively.
A US Investigational New Drug Application was approved for the IMPACT programme in August 2023 and the Company plans to dose the first patients once funding is secured.

PROACT Programme: Muscle Optimisation in Obesity
In view of its multi-modal pharmacology, S-pindolol benzoate is an obvious candidate to evaluate in this setting. In 2024, Actimed initiated a new development programme investigating the potential benefits of using S-pindolol benzoate (ACM-001.1) during and post-GLP-1 receptor agonist (GLP-1 RA) therapy in the management of obesity and related metabolic conditions.
This new initiative comprises a preclinical and clinical programme:
- A preclinical programme, designed to explore the pharmacological mechanisms, safety and efficacy of S-pindolol benzoate in a diet-induced model of obesity in animals who are receiving or who have received a GLP-1 RA. The in-vivo phase of these studies has completed confirming that S-pindolol benzoate is worthy of further investigation in a clinical programme.
- PROACT (Preserving, Restoring, and Optimising (lean mass and muscle) with ACTAs) – a two-part Phase 2a, randomised, placebo controlled clinical trial which will assess the safety and efficacy of S-pindolol benzoate in obese patients 1) during and 2) post-GLP-1 RA therapy. The first patients were enrolled in June 2025 and initial results are expected in the second half of 2026. This study will include up to 48 patients

Mode of action
S-pindolol has been described as an anabolic/catabolic transforming agent1 with a multifunctional effect on three key pharmacological targets relevant for cachexia and muscle wasting:
• Reduction of catabolism via β-1 receptor antagonism3,4,5
• Increased anabolism and muscle growth, through partial β2 receptor agonism1
• Improvement in appetite and fatigue via central 5-HT1a antagonism6,7,8,9,10,11
This trio of anti-catabolic and pro-anabolic pharmacology makes S-pindolol a highly promising candidate for development in muscle wasting diseases.
Clinical data
The ACT-ONE Phase 2 clinical study of S-pindolol was a proof of concept exploratory, randomised, double-blind, placebo-controlled study in 87 patients with non-small cell lung cancer (NSCLC) or colorectal cancer (CRC). The trial demonstrated that S-pindolol up to 10 mg twice per day (BID) was well-tolerated and that patients receiving 10 mg BID S-pindolol showed a statistically significant median weight gain of 2.74kg compared to a median weight loss of 1.09 kg in patients receiving placebo over a 4-month period2.
A statistically significant improvement in the functional outcome of Handgrip Strength (HGS) was also demonstrated in patients receiving S-pindolol (10 mg BID).
Actimed has completed a Phase 1 Pharmacokinetic and Pharmacodynamic (PK/PD) comparative bioavailability study (NCT06028321) of S-pindolol benzoate in healthy subjects. This study was a comparison of S-pindolol benzoate to the authorised product racemic pindolol and investigated pharmacokinetics across a range of doses12.
The study demonstrated that S-pindolol when administered as the single enantiomer, was essentially bioequivalent to an equivalent dose of S-pindolol when administered as part of a racemic mixture of pindolol (the authorised product). The study also demonstrated the lack of in vivo racemization of S-pindolol to R-pindolol, with dose-dependent pharmacokinetics after single and multiple doses.
Patent portfolio
Actimed has built an extensive IP portfolio that provides robust intellectual property protection supporting Actimed’s lead asset, ACM001.1 (S-pindolol benzoate).
In 2024, Actimed received patent grants from the European Patent Office and the United States Patent and Trademark Office. European Patent EP 4132909 B covers a pharmaceutically acceptable salt of S-pindolol and benzoic acid, with expiry in 2041. US Patent US 12,109,192 B covers the two preferred crystalline forms of S-pindolol benzoate, with expiry in 2041.
Reference
1Pötsch MS, Tschirner A, Palus S, von Haehling S, Doehner W, Beadle J, Coats AJ, Anker SD, Springer J. The anabolic catabolic transforming agent (ACTA) espindolol increases muscle mass and decreases fat mass in old rats. J Cachexia Sarcopenia Muscle.. 2014 Jun;5(2):149-58
2A Coats et al Espindolol for treatment and prevention of cachexia: the ACT-ONE trial. J Cachexia Sarcopenia Muscle 2016; 7: 355–365
3Clark BJ and Bertholet A. (1983). Effects of pindolol on vascular smooth muscle. Gen. Pharmacol.; 14: 117-119
4Jeppsson AB, Johansson U and Waldeck B. (1984). Steric aspects of agonism and antagonism at betaadrenoreceptors: experiements with the enantiomers of terbutaline and pindolol. Act. Pharmacol. Toxicol
(Copenh).; 54(4): 285-291
5Boucher M, Duchene-Marullaz P and Moundanga JL. (1986). Studies on the stereoisomers of betaadrenoreceptor
antagonists in conscious A-V blocked dogs. Br. J. Pharmacol.; 89(1): 119-127
6Newman-Tancredi A, Chaput C, Gavaudan S, Verriele L, Millan MJ. Agonist and antagonist actions of (-) pindolol, at recombinant, human serotonin 1A (5-HT1A) receptors. Neuropsychopharmacology. 1998 May; 18(5):395-398
7Andree B, Thorburg SO, Halldin C, Farde L. Pindolol binding to 5-HT1a receptors in the human brain confirmed with positron emission tomography. Psychopharmacology (Berl). 1999 June; 144(3) 303-305
8Martinez D, Hwang D, Mawlawi O, Slifstein M, Kent J, Simpson N, Parsey RV, Hashimoto T, Huang Y, Shinn A, Van Heertum R, Abi-Dargham A, Caltabiano S, Malizia A, Cowley H, Mann JJ, Laruelle M. Differential occupancy of somatodendritic and postsynaptic 5HT(1A) receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology. 2001 Mar;24(3):209-29
9Castro ME, Harrison PJ, Pazos A, Sharp T. Affinity of (+/-)-pindolol, (-)-penbutolol, and (-)-tertatolol for pre- and postsynaptic serotonin 5-HT(1A) receptors in human and rat brain. J Neurochem. 2000 Aug;75(2):755-62
10Yan H and Lewander T. Differential tissue distribution of the enantiomers of racemic pindolol in the rat. Eur Neuropsychopharmacol. 1999 Dec; 10(1): 59-62
11Olver Js, Cryan JF, Burrows GD, Norman TR. Pindolol augmentation of antidepressants: a review and rationale. Aust. NZ. J. Psychiatry 2000 Feb;34 (1): 71-79
12Pharmacokinetics, Pharmacodynamics and Bioavailability of ACM-001.1 (S-Pindolol Benzoate) in Healthy Volunteers. Misselwitz, F., Henderson, D., Menakuru, S., Morten, E., Roe, C., Whitaker, G., Wohlfeil, S. and McDermott, J. (2024). The publication may be accessed
trademark registration number 6734037 in the USA

